It is a heat-resistant and high-strength material with various features including hardness,
good heat resistance, and good heat conductance.
Silicon carbide (SiC) is a covalent compound rarely found in nature, wherein carbon (C) and silicon (Si) are bound to each other on a one-to-one basis.
SiC has high hardness, good heat resistance, and good heat conductance and is therefore used as an abrasive and a refractory. Furthermore, it has received a lot of attention recently as a material for semiconductors, etc. because of its exceptional heat resistance at high temperatures.
Outstanding heat resistance
 Silicon carbide (SiC) is highly resistant to heat. It is stable up to temperatures near 1,600 °C in air, and its decomposition temperature is 2,545 °C. Particularly notable is its exceptional resistance to high temperatures. (*1)
Silicon carbide (SiC) is highly resistant to heat. It is stable up to temperatures near 1,600 °C in air, and its decomposition temperature is 2,545 °C. Particularly notable is its exceptional resistance to high temperatures. (*1)
[Refractoriness of SiC and other ceramics]
| Name |
Refractoriness |
| Crystalline alumina |
› SK40 mp. 2,050 °C |
| Fused alumina |
› SK40 mp. 2,050 °C |
| Fused silica |
› SK32 mp. 1,720 °C |
| Silicon carbide |
› SK40 Decomposition temperature 2,545 °C |
| Silicon nitride |
SK38 Decomposition temperature 1,850 °C |
*SK number: a number assigned to indicate the temperature at which pyrometric cones soften and bend. SK32 is 1,710 °C; SK38 is 1,850 °C; and SK40 is 1,920 °C.
Good heat conductance
 SiC is a good conductor of heat. Sintered bodies have a thermal conductivity of 270 W/m·K, which is comparable to the metalloids. (*1)
SiC is a good conductor of heat. Sintered bodies have a thermal conductivity of 270 W/m·K, which is comparable to the metalloids. (*1)
[Thermal conductivity of silicon carbide and other ceramics & metals (W/(m·k))]
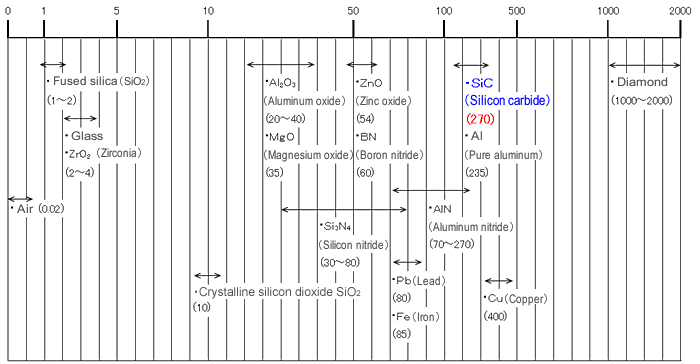
Note: numbers in parentheses are thermal conductivity values.
See thermal conductivity values, document values.
Semiconductor properties
 SiC is a semiconductor with an electric resistance that undergoes changes greater than 10 figures, ranging from a zone of resistance at which it can be used as a heating element to a zone of near insulation. Further, research and development is actively proceeding on a power device such as a high-temperature semiconductor which can be used up to approximately 500 °C due to its high electric field for insulation breakdown and high heat conductance with a wide bandgap (two or three times wider than Si). (*1)
SiC is a semiconductor with an electric resistance that undergoes changes greater than 10 figures, ranging from a zone of resistance at which it can be used as a heating element to a zone of near insulation. Further, research and development is actively proceeding on a power device such as a high-temperature semiconductor which can be used up to approximately 500 °C due to its high electric field for insulation breakdown and high heat conductance with a wide bandgap (two or three times wider than Si). (*1)
Good chemical resistance
 Silicon carbide (SiC) is chemically stable, and resists corrosion by acids and bases. It is unaffected by acid mixtures of heated hydrofluoric acid plus nitric acid, nor by concentrated sodium hydroxide. It can, however, be broken down by a melting method using sodium carbonate, or by a high-pressure acid decomposition method using a mixture of hydrofluoric acid, nitric acid and sulfuric acid.
Silicon carbide (SiC) is chemically stable, and resists corrosion by acids and bases. It is unaffected by acid mixtures of heated hydrofluoric acid plus nitric acid, nor by concentrated sodium hydroxide. It can, however, be broken down by a melting method using sodium carbonate, or by a high-pressure acid decomposition method using a mixture of hydrofluoric acid, nitric acid and sulfuric acid.


